Incorporated into the theme of ISPOR Europe 2022 is the notion of “building and using evidence to enable access.” Flatiron’s presence at this year’s leading global scientific conference for Health Economics and Outcomes Research (HEOR) highlights our ability to do just that, develop and evaluate EHR-derived RWE for value and access.
We showcase the value of integrated evidence through a study using linked EHR-Claims data to provide broader longitudinal understanding of patient journeys, mitigate data missingness, and control for confounding from a single source RWD. EHR-Claims linked datasets have the opportunity to unlock a variety of research topics - as seen in our study on comorbidities and survival in people with cancer.
Further, we demonstrate how access to radiologic images creates a novel type of RWD available in HEOR. Cohorts of patients with cancer imaging scans are compared to a broader real-world population in a key study to assess and establish the representativeness of imaging data for research.
We are thrilled to present our research exploring breakthrough innovations in machine learning (ML) to efficiently extract information from patient charts and generate variables for analysis. New research findings demonstrate when and how ML can be fit-for-purpose in comparative-effectiveness research. Dr. Corey Benedum speaks on-stage at the ISPOR Europe Issue Panel “Real-World Data at Scale: How Machine Learning Can Enable Learning from All Patients” as well as an oral podium presentation of findings from an extensive research replicating HEOR analyses with ML-extracted variables, titled “Machine learning-accelerated outcomes research: A case study of cancer biomarker associated survival”.
These exciting advancements in EHR-Claims data linkages, radiology images, and ML-extraction techniques are empowering us to learn from a deeper and broader number of patient experiences than has ever been possible before. When combined with rigorous analytic methods, we are able to reimagine the infrastructure of health care and health policy by enabling the scale, depth, and inference for high quality RWE. We are committed to using data for good - understanding the value of new medicines and increasing access around the world.
Comparison of comorbidity indices between EHR-derived database and claims data among patients with metastatic breast cancer
Authors: Alemseged Ayele Asfaw, et al.
Overview
Baseline patient characteristics relevant to cancer diagnosis and treatment are more likely to be captured in an oncology EHR. In contrast, information captured from claims data likely includes more patient information beyond the specific cancer diagnosis.
In this study, researchers compared individual comorbidities and Charlson Comorbidity Index (CCI) derived from both EHR and claims data in patients with HR+/HER2- metastatic breast cancer to assess comorbidity data comparability between the two sources.
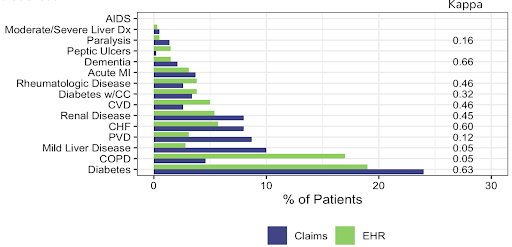
Overall, CCI component comorbidities showed comparable prevalence across data sources with notable differences in COPD and mild liver disease.
In addition, similar hazards of mortality were observed for CCI scores of 0, 1 and 2+ between the data sources.
Why this matters
EHR records and healthcare claims are collected primarily for clinical care and healthcare services reimbursement, respectively.
While clinicians directly document comorbidities in an EHR, the data can be limited, making it difficult to accurately include/exclude patients with comorbidities or adjust for this in research. Using both data sources, researchers can maximize information when selecting patients with CCI comorbidities to enhance their research
View the full poster on the ISPOR website
Association between antibiotic exposure and survival among patients diagnosed with advanced melanoma or advanced non-small cell lung cancer (NSCLC) treated with Immune Checkpoint Inhibitor (ICI) therapy
Authors: Samantha Reiss, et al.
Overview
Previous studies reviewing the association between antibiotic exposure and survival in patients with cancer treated with ICI therapy suggest poor outcomes. Using an EHR-claims linked dataset, researchers investigated this further in a large cohort of patients diagnosed with advanced melanoma or NSCLC receiving ICI therapy.
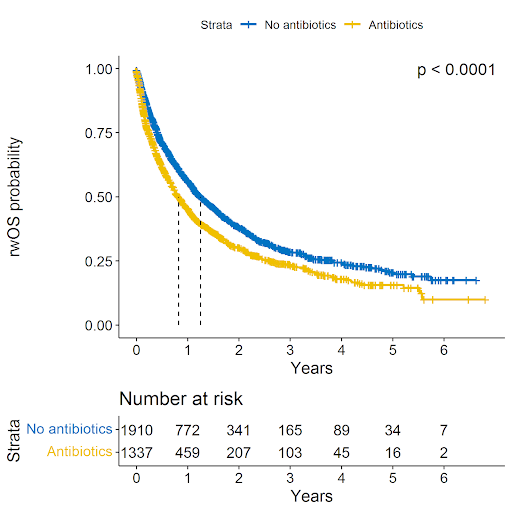 Exposure to antibiotics around the time of ICI initiation, as identified by the presence or absence of at least one systemic antibiotic use claim, is associated with worse real-world overall survival of patients with aMel and aNSCLC treated with ICI therapy. The adjusted and weighted hazard ratio for mortality is 1.26 (CI 1.15 - 1.38, p<0.001)
Exposure to antibiotics around the time of ICI initiation, as identified by the presence or absence of at least one systemic antibiotic use claim, is associated with worse real-world overall survival of patients with aMel and aNSCLC treated with ICI therapy. The adjusted and weighted hazard ratio for mortality is 1.26 (CI 1.15 - 1.38, p<0.001)
Why this matters
This study included a large patient cohort, and a novel integrated EHR-claims linked data set to identify claims-derived drug exposure and determine associations between non-oncology drug exposure and EHR-derived oncology outcomes.
View the full poster on the ISPOR website
Bias characterization of real-world patients with and without imaging in a community oncology electronic health records database
Authors: Xinye Li, et al.
Overview
Selecting real-world patients based on the availability of real-world radiographic imaging data may introduce biases, considering the difference in its timing/availability at the patient level, thus potentially affecting the generalizability of results. This study aimed to assess the representativeness of imaging-derived cohorts relative to two broad real-world study cohorts, patients with advanced non-small cell lung cancer (aNSCLC) and patients with diffuse large B-cell lymphoma (DLBCL).
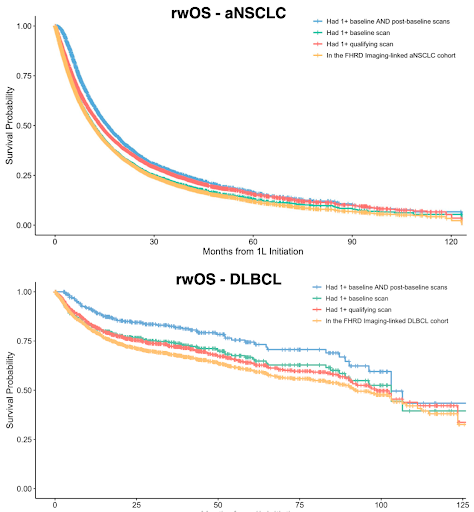 For both the aNSCLC and DLBCL cohort, patients with scans at any time or in baseline window were broadly representative to the respective target. However, requiring scans at both baseline and post-baseline windows resulted in some covariate and outcome differences with the target cohort.
For both the aNSCLC and DLBCL cohort, patients with scans at any time or in baseline window were broadly representative to the respective target. However, requiring scans at both baseline and post-baseline windows resulted in some covariate and outcome differences with the target cohort.
Why this matters
Radiographic imaging data is critical in clinical decision-making because it clearly shows cancer diagnosis and progression. As a result, imaging data complements EHR data in providing a more holistic view of a patient's real-world experience.
For each respective disease, this study focused on three different sets of patients with scans available at various time points following receipt of first-line (1L) treatment: any time point, baseline (pre-1L initiation), baseline and post-baseline (during 1L duration).
The study found that requiring scans at any time or in the baseline window resulted in the cohort being representative relative to the broader real-world cohort; however, requiring scans in the post-baseline window may introduce selection bias and immortal time bias.
View the full poster on the ISPOR website
Can ML-extracted variables reproduce real world comparative effectiveness results from expert-abstracted data? A case study in metastatic non-small cell lung cancer treatment
Authors: Arjun Sondhi, et al.
Overview
Machine learning (ML) extraction of clinical characteristics from unstructured text in electronic health records is more cost-effective and scalable when compared to manual abstraction. This study determined if ML-extracted variables are fit for research use and can effectively reproduce scientific conclusions compared to expert-abstracted data.
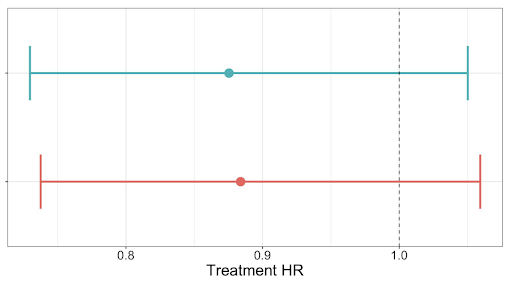 When estimating the hazard ratios for real world Overall Survival (rWOS) with 95% CIs, neither result shows a significantly significant difference in OS between treatment groups, and have highly overlapping confidence intervals.
When estimating the hazard ratios for real world Overall Survival (rWOS) with 95% CIs, neither result shows a significantly significant difference in OS between treatment groups, and have highly overlapping confidence intervals.
Top: abstracted cohort, bottom: ML-extracted cohort.
Why this matters
It is well-known how burdensome and resource-intensive manual abstraction from unstructured documents is.
ML tools have emerged as a valuable approach to automate this extraction of information; however, it is essential to evaluate ML-extracted variables in the context of data quality to understand their reliability and potential for error better.This research demonstrated that oncology RWD extracted using high-performing ML models may be used as an alternative to expert-abstraction.